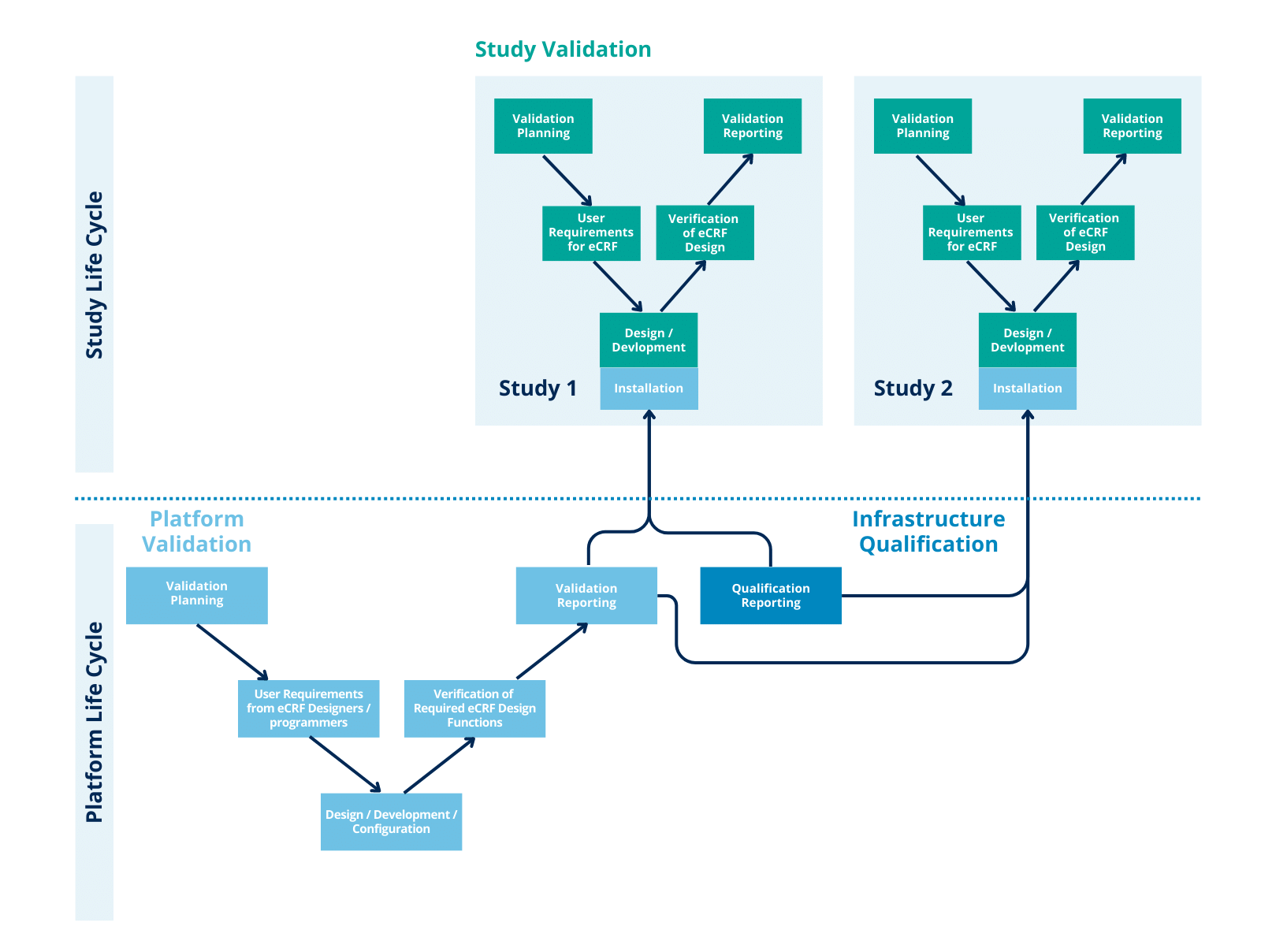
Validation Of Laboratory Computerized Systems . recently updated to conform with gamp® 5 concepts and terminology, as well as recent regulatory and industry developments, the ispe gamp® good. the approach to validation should be based on a risk assessme nt that takes into consideration the intended use of the. describes a flexible categorization approach consistent with gamp® 5, based on risks associated with the use of the system to support the relevant. validation of software and computer systems • help with design qualification, e.g., provide. this guideline defines basic principles for the validation of computerised systems used within official medicines control. the aim of this document is to provide guidance on the validation of computerised systems, compliant with good laboratory.
from qbdgroup.com
validation of software and computer systems • help with design qualification, e.g., provide. the aim of this document is to provide guidance on the validation of computerised systems, compliant with good laboratory. describes a flexible categorization approach consistent with gamp® 5, based on risks associated with the use of the system to support the relevant. this guideline defines basic principles for the validation of computerised systems used within official medicines control. the approach to validation should be based on a risk assessme nt that takes into consideration the intended use of the. recently updated to conform with gamp® 5 concepts and terminology, as well as recent regulatory and industry developments, the ispe gamp® good.
Computerized System Validation in Clinical Trials Key Considerations
Validation Of Laboratory Computerized Systems describes a flexible categorization approach consistent with gamp® 5, based on risks associated with the use of the system to support the relevant. the aim of this document is to provide guidance on the validation of computerised systems, compliant with good laboratory. describes a flexible categorization approach consistent with gamp® 5, based on risks associated with the use of the system to support the relevant. recently updated to conform with gamp® 5 concepts and terminology, as well as recent regulatory and industry developments, the ispe gamp® good. this guideline defines basic principles for the validation of computerised systems used within official medicines control. the approach to validation should be based on a risk assessme nt that takes into consideration the intended use of the. validation of software and computer systems • help with design qualification, e.g., provide.
From gmptrends.com
Laboratory Computer System Validation GMP Trends Validation Of Laboratory Computerized Systems this guideline defines basic principles for the validation of computerised systems used within official medicines control. the approach to validation should be based on a risk assessme nt that takes into consideration the intended use of the. validation of software and computer systems • help with design qualification, e.g., provide. the aim of this document is. Validation Of Laboratory Computerized Systems.
From www.collidu.com
Computer System Validation PowerPoint and Google Slides Template PPT Validation Of Laboratory Computerized Systems recently updated to conform with gamp® 5 concepts and terminology, as well as recent regulatory and industry developments, the ispe gamp® good. describes a flexible categorization approach consistent with gamp® 5, based on risks associated with the use of the system to support the relevant. the aim of this document is to provide guidance on the validation. Validation Of Laboratory Computerized Systems.
From pflb.us
Computer System Validation (CSV) in Pharma Industry Process, Steps Validation Of Laboratory Computerized Systems recently updated to conform with gamp® 5 concepts and terminology, as well as recent regulatory and industry developments, the ispe gamp® good. the approach to validation should be based on a risk assessme nt that takes into consideration the intended use of the. the aim of this document is to provide guidance on the validation of computerised. Validation Of Laboratory Computerized Systems.
From www.chromatographyonline.com
GAMP Good Practice Guide for Validation of Laboratory Computerized Validation Of Laboratory Computerized Systems recently updated to conform with gamp® 5 concepts and terminology, as well as recent regulatory and industry developments, the ispe gamp® good. this guideline defines basic principles for the validation of computerised systems used within official medicines control. the aim of this document is to provide guidance on the validation of computerised systems, compliant with good laboratory.. Validation Of Laboratory Computerized Systems.
From www.axians.ch
Computerized System Validation Axians CH Validation Of Laboratory Computerized Systems describes a flexible categorization approach consistent with gamp® 5, based on risks associated with the use of the system to support the relevant. this guideline defines basic principles for the validation of computerised systems used within official medicines control. validation of software and computer systems • help with design qualification, e.g., provide. the aim of this. Validation Of Laboratory Computerized Systems.
From www.getreskilled.com
What is Computer System Validation (CSV) in Pharma? Validation Of Laboratory Computerized Systems describes a flexible categorization approach consistent with gamp® 5, based on risks associated with the use of the system to support the relevant. the approach to validation should be based on a risk assessme nt that takes into consideration the intended use of the. validation of software and computer systems • help with design qualification, e.g., provide.. Validation Of Laboratory Computerized Systems.
From bncengineers.com
Computer system validation Industrial Automation Validation Of Laboratory Computerized Systems the aim of this document is to provide guidance on the validation of computerised systems, compliant with good laboratory. recently updated to conform with gamp® 5 concepts and terminology, as well as recent regulatory and industry developments, the ispe gamp® good. validation of software and computer systems • help with design qualification, e.g., provide. this guideline. Validation Of Laboratory Computerized Systems.
From pharmsolution.org
Computerized system validation Pharma Solutions Ltd Validation Of Laboratory Computerized Systems the aim of this document is to provide guidance on the validation of computerised systems, compliant with good laboratory. recently updated to conform with gamp® 5 concepts and terminology, as well as recent regulatory and industry developments, the ispe gamp® good. validation of software and computer systems • help with design qualification, e.g., provide. describes a. Validation Of Laboratory Computerized Systems.
From 34.225.221.91
Computer System Validation (CSV) in Clinical Trials Integra IT Validation Of Laboratory Computerized Systems recently updated to conform with gamp® 5 concepts and terminology, as well as recent regulatory and industry developments, the ispe gamp® good. the aim of this document is to provide guidance on the validation of computerised systems, compliant with good laboratory. the approach to validation should be based on a risk assessme nt that takes into consideration. Validation Of Laboratory Computerized Systems.
From www.chromatographyonline.com
GAMP Good Practice Guide for Validation of Laboratory Computerized Validation Of Laboratory Computerized Systems validation of software and computer systems • help with design qualification, e.g., provide. the aim of this document is to provide guidance on the validation of computerised systems, compliant with good laboratory. describes a flexible categorization approach consistent with gamp® 5, based on risks associated with the use of the system to support the relevant. recently. Validation Of Laboratory Computerized Systems.
From www.slideserve.com
PPT Validation of Computer Systems & Software A Practical Approach Validation Of Laboratory Computerized Systems describes a flexible categorization approach consistent with gamp® 5, based on risks associated with the use of the system to support the relevant. validation of software and computer systems • help with design qualification, e.g., provide. recently updated to conform with gamp® 5 concepts and terminology, as well as recent regulatory and industry developments, the ispe gamp®. Validation Of Laboratory Computerized Systems.
From www.adeodata.eu
Validation & Data Integrity Adeodata Validation Of Laboratory Computerized Systems the approach to validation should be based on a risk assessme nt that takes into consideration the intended use of the. describes a flexible categorization approach consistent with gamp® 5, based on risks associated with the use of the system to support the relevant. this guideline defines basic principles for the validation of computerised systems used within. Validation Of Laboratory Computerized Systems.
From pflb.us
Computer System Validation (CSV) in Pharma Industry Process, Steps Validation Of Laboratory Computerized Systems the approach to validation should be based on a risk assessme nt that takes into consideration the intended use of the. validation of software and computer systems • help with design qualification, e.g., provide. describes a flexible categorization approach consistent with gamp® 5, based on risks associated with the use of the system to support the relevant.. Validation Of Laboratory Computerized Systems.
From www.pharmamirror.com
How Does Computer System Validation Help Pharmaceutical Quality Validation Of Laboratory Computerized Systems validation of software and computer systems • help with design qualification, e.g., provide. recently updated to conform with gamp® 5 concepts and terminology, as well as recent regulatory and industry developments, the ispe gamp® good. this guideline defines basic principles for the validation of computerised systems used within official medicines control. the aim of this document. Validation Of Laboratory Computerized Systems.
From www.k2c.com
Computerised System Validation Validation Of Laboratory Computerized Systems validation of software and computer systems • help with design qualification, e.g., provide. describes a flexible categorization approach consistent with gamp® 5, based on risks associated with the use of the system to support the relevant. this guideline defines basic principles for the validation of computerised systems used within official medicines control. the approach to validation. Validation Of Laboratory Computerized Systems.
From fivevalidation.com
O que é Validação de Sistemas Computadorizados? O que é GAMP 5 Validation Of Laboratory Computerized Systems the aim of this document is to provide guidance on the validation of computerised systems, compliant with good laboratory. recently updated to conform with gamp® 5 concepts and terminology, as well as recent regulatory and industry developments, the ispe gamp® good. the approach to validation should be based on a risk assessme nt that takes into consideration. Validation Of Laboratory Computerized Systems.
From onestepsolutionz.com
Computerized System Validation (CSV) 1Step Healthcare Validation Of Laboratory Computerized Systems validation of software and computer systems • help with design qualification, e.g., provide. this guideline defines basic principles for the validation of computerised systems used within official medicines control. the approach to validation should be based on a risk assessme nt that takes into consideration the intended use of the. describes a flexible categorization approach consistent. Validation Of Laboratory Computerized Systems.
From ecvalidation.com
Validation of Computerized Systems in a Pharmaceutical Wholesaler Validation Of Laboratory Computerized Systems validation of software and computer systems • help with design qualification, e.g., provide. recently updated to conform with gamp® 5 concepts and terminology, as well as recent regulatory and industry developments, the ispe gamp® good. this guideline defines basic principles for the validation of computerised systems used within official medicines control. the aim of this document. Validation Of Laboratory Computerized Systems.